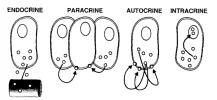
This is also the basis for my having delved pretty deeply [and continuing to do so] with regard to DHEA: it is highly sexually dimorphic in its effects, meaning it behaves so differently in men versus women, and in different tissues.
A lot of the reason that DHEA surprises people with how potently it reverses crashed E2 relates to intracrinology. DHEA acts, as an adrenal steroid, primarily in peripheral tissues. So its aromatization is rapid and in the local tissues rather than primarily in the blood.
You can read a bit more about the differences between intracrinology and endocrinology and how DHEA relates here:
From Labrie, Fernand. “Intracrinology.”
Molecular and Cellular Endocrinology, vol. 78, no. 3, July 1991, pp. C113–18.
DOI.org (Crossref),
Redirecting.
Annotations
“A large proportion of androgens in men (40%), and the majority of estrogens in women (75% before menopause and close to 100% after menopause), are synthesized in peripheral target tissues from precursor steroids of adrenal origin.” (Labrie, 1991, p. 13)
View attachment 350261(Labrie, 1991, p. 14)
“Rule of adrenal precursor sex steroids @TS” (Labrie, 1991, p. 14)
“A”-dione. DHEA and DHEA-S should not be called adrenal” (Labrie, 1991, p. 14)
View attachment 350262(Labrie, 1991, p. 14)
““androgens”, since these three steroids possess no intrinsic biological activity, and require transformation by steroidogenic enzymes in peripheral target tissues to become active androgens or estrogens (Fig. 2). We thus propose to call these three steroids precursor sex steroids (PSS), a term which more adequately describes their role and does not presume their final biological activity.” (Labrie, 1991, p. 15)
“Sources of androgens in men As a measure of the importance of adrenal precursor sex steroids in adult man, serum levels of the main metabolites of androgens - 5aandrostane-3a,l7P-diol (3a-dial), androsterone (ADT) and their glucuronidated derivatives, 3adiol-G and ADT-G - are reduced only by 5070% following surgical or medical castration (Moghissi et al., 19841, evidence that the conversion of adrenal precursor sex steroids accounts for 30-50% of total androgens in adult men.” (Labrie, 1991, p. 15)
“we have developed a combination therapy where the formation of androgens by the testes is blocked by the administration of an LHRH agonist or surgical castration while, at the same time, the action of androgens of adrenal origin is blocked in prostatic tissue by administration of the pure antiandrogen flutamide. Blockade of adrenal androgens with antiandrogen is the first treatment demonstrated in large scale studies to prolong life in prostate cancer (Labrie et al., 1985; B&land et al., 1987; Crawford et al., 1989). The combination therapy is now used worldwide and is being extended to early stages of the disease in order to facilitate radical prostatectomy and radiotherapy (Monfette et al., 1990).” (Labrie, 1991, p. 16)
“blockade of androgens to obtain compounds with higher potency at the level of the androgen receptor and to block androgen formation by 17P-HSD and Sa-reductase. Blockade of 17P-HSD and Scu-reductase activity should have no serious systemic side effects since glucocorticoid and mineralocorticoid pathways would remain intact. Such compounds present clear advantages over less specific inhibitors of steroidogenesis, such as aminoglutethimide and ketoconazole, which inhibit cortisol secretion; they would thus avoid the necessity of replacement therapy with glucocorticoids (and sometimes mineralocorticoids).” (Labrie, 1991, p. 16)
“Sources of estrogens in women In women, the role of the adrenal precursors DHEA-S, DHEA and A”-dione in the peripheral formation of estrogens is likely to be even more important than that described for the formation of androgens from the same precursors in men. The best estimate of the intracrine formation of estrogens in peripheral tissues in women is in the order of 75% before menopause, and close to 100% after menopause.” (Labrie, 1991, p. 16) Synthesis of sex steroids (!) estrogen (!) included is primarily from adrenal origin (75%+) in women!
And for a topical discussion relating to the pitfalls of relying on bloodwork levels to describe tissue-level estrogen regulation, refer to
Primobolan / Equipoise Crashed my E2 – Help! and its section titled:
Limitations of Circulating Levels as an Index of Tissue-Specific Estrogen Regulation
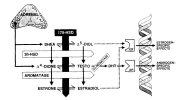
[10]
AD: androstenedione
Regulation of estrogen production and metabolism within peripheral tissues is enabled by local expression of Aromatase (CYP19A1), which converts androgens to estrogens (T ⇒ E2 and AD ⇒ E1 [E2 is the most prevalent estrogen in men; which may explain the greater tolerability for EQ in women]). Estrogens further can be converted to estrogen sulfates and estrogen fatty acyl esters via Estrogen Sulfotransferase (EST) and Acyl-Transferases, respectively. Finally, these estrogen derivatives can be converted back to parent estrogens by Steroid Sulfatase (Sulfatase) and Lipase activity [10].
Adipose Tissue (AT) is particularly enriched in estrogen fatty acyl esters and consequently has an extensive buffering system that enables local regulation of estrogen production and metabolism… Notably, in a study of obese men, E2
fatty acyl ester concentrations did correlate in serum and fat (Wang, et al., 2013) [10], possibly indicating that serum estrogen levels influence stored estrogen content in AT, but conversion to bioactive forms is locally regulated [10].
Several clinical studies have demonstrated dissociations between circulating and intra-adipose estrogen levels, including in men (Blankenstein, et al., 1992; Belanger, et al., 2006; Deslypere, et al., 1985; Wang, et al., 2013) [10].