Ghoul
Member
Alright... so apparently it's not great to have this stuff in our vials. I've just ordered some Tirz15 from SRY and wondering if I made a mistake here. I guess it's benign to inject but it might cause aggregates when I keep it reconstituted for "too long"? How would I know it went bad? Cloudy?
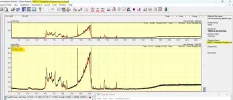
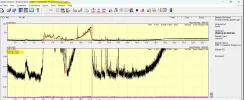
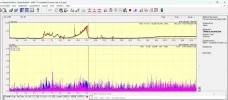
Cloudy means the aggregates are large enough to be visible, which is huge, at least 100um per spec in the "cloud". That's tens of trillions of individual monomers per visible spec. Below that size they're invisible.
While time can be a factor when aggregation is caused by excess concentration, heat, light, etc, in a case like this where the excipient immediately damages the monomers, aggregates typically don't "grow", but spontaneously form instantly into their maximum size. So you can safely filter into another vial and not be too concerned about the aggregates "regrowing" (which can happen with other causes of aggregation).
The larger the aggregate, the more of a concern they are. 10um to 100um is the size range considered most risky.
If you use a .22um sterile syringe PES filter, you'll eliminate the vast majority of that range or larger and make the Tirz as safe as possible. As a bonus you'll sterilize it as well, since many peptides are unsterile.
PS: Most of the time aggregates won't be visible, the riskiest size range is sub-visible, so filtering everything is best practice imo.