bc1qt9cz55heqn42uckfuadfgzwjtm2kfugafmwkzjHow can we contribute to the fund?
Btc address of the test fund! Appreciate your interest
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
bc1qt9cz55heqn42uckfuadfgzwjtm2kfugafmwkzjHow can we contribute to the fund?
do you have any better search terms than project 3. im kinda lazyProject 3
do you have any better search terms than project 3. im kinda lazy
do you have any better search terms than project 3. im kinda lazy
do you have any better search terms than project 3. im kinda lazy
do you have any better search terms than project 3. im kinda lazy
bc1qt9cz55heqn42uckfuadfgzwjtm2kfugafmwkzj
Btc address of the test fund! Appreciate your interest
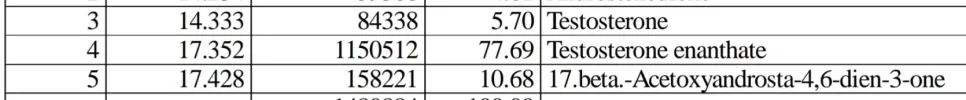
The GCMS and HPLC results on the C6-C7 -ene contamination of TC/TE raw samples demonstrated the situation clearly.
Yep go back to the thread and we addressed that part. Good point.View attachment 353392
i think this is a good example of gcms being unreliable in autoid.
i dont think its really that unreliable at all with the recent gc-ms tests we had with jano. common pattern. especially not at these crazy area %
Ok so here's some analysis:what the hell, old raw only tested as test and delta 6 test
we have went over delta 6 test which is completely useless making him off with a 76% raw which is a big problem.
but now we're back to my first guess. you have benzoyl solvents(Acrylophenone) and and synthetic impurities in there(Methylbenzenesulfonyloxy)
this is absolutely unacceptable if this were the pure raw
Based on the majority of Jano testing, the gcms appears to be fairly accurate. On the other hand, it seems like the hplc has good differentiation with anything that is more than a single hydrogen apart. Although do note that the chance of Jano being able to test proteins like HGH accurately is quite slim. Minute differences or missfolding would likely not show up.HPCL of my sample
What do you mean by this statement? Could you elaborate please?Based on the majority of Jano testing, the gcms appears to be fairly accurate.
Meh, I'm too far outta my league trying to follow this one.Not sure what you mean by that
How did you come to this conclusion?On the other hand, it seems like the hplc has good differentiation with anything that is more than a single hydrogen apart.
also interested, because that isnt true when looking the gcms through.How did you come to this conclusion?
I'm probably just missing something obvious. Great to learn on here from folks who have real world experience and knowledge.also interested, because that isnt true when looking the gcms through.
100% im the least experienced here so great to pick up some info.I'm probably just missing something obvious. Great to learn on here from folks who have real world experience and knowledge.
Very important typo. I meant to say fairly inaccurate. Thank you @readalot for bringing this to my attention.Based on the majority of Jano testing, the gcms appears to be fairly accurate.
Long story short, I decided to do some basic statistics. Note the uncofindence in my statement — these kinds of statements are closer to "more likely than not" rather than "95% confidence". This is mainly due to the small sample size (~10) more than anything. Again, the conclusion is supposed to be that GCMS area is not accurate rather than is.What do you mean by this statement? Could you elaborate please?
This is a bit less scientific, and is mostly a combination of: how likely two molecules are to coelute based on structure (in the case of d4,6, I would say the damning aspect is that the double bond is in very close proximity to the d4 bond, unlike modifications far away from it like boldenone or nandrolone) and published data, how the hplc raw data looks (especially the chromatograms, I actually think that I saw a shoulder peak on one), how it compares to the gcms, etc. Its a bit hard to explain, but at minimum I haven't seen anything nearly as flagrant as the d4,6 and testosterone.How did you come to this conclusion?
i actually wrote to readalot in dms and did mention making a database for it in google spreadsheet or whatever, but i gave up fairly quickly because we have less than 5 tests total with hplc & gcms in raws on the forum. sadly no one does it.with gcms and hplc. If we can get enough data, we could even do cluster analysis and find patterns with Jano's testing, possibly even with vendors.
NPP by QSC two samples November 2024
I have paid for the purity test do you feel like it would help the cause if we use the meso fund to get GCMS on both?

