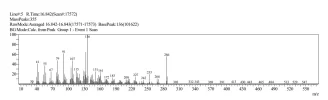
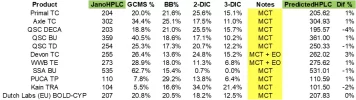
Janoshik is saying is that there are 2 main possibilities. One is that the temperature used during GCMS oxidized the 6 position carbon (a spot that is quite sensitive) of ~10% of the testosterone, causing a delta 4,6. See AASraw thread for specifics of this molecule. The other option is that while manufacturing, 10% already existed and that hplc failed to notice the minute difference (litteraly 1 H apart).
As a chemist, I would say that the latter is much more likely. First, I'd expect that the spectra of the 2 molecules to be very similar and might coelute on hplc -> meaning that it's basically impossible to distinguish on most hplc machines. On the other hand, the chance of oxidation at that one specific position, due to heat, is unlikely. Usually, you would expect polymerization or many different compounds when thermally oxidizing. A catalyst could make this more likely, but many such catalysts are either basics/acids or platinum group metals, which is unlikely to be in the hplc machine.
If you want to verify this, have
@janoshik retest. Specifically, take the exact sample and heat it to 250C or whatever temp his gcms machine is. Leave it for an hour or so, and retest. If the amount of this specific analog increases, then it is likely your culprit. If nothing happens or other components increase, then it was likely an impurity - likely from over-oxidation while forming the double bond. Not sure how much this'd cost but that is an option.