Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
Style variation
Guest viewing is limited
- You have a limited number of page views remaining
- 1 guest views remaining
- Register now to remove this limitation
- Already a member? Click here to login
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
1ml enough for 36IU
- Thread starter Laworg
- Start date
Solution
what syringes do you use to inject the bac water and do you need to change the needle every time you draw and extract the water?
I use 25g 1” needles and 3ml (luer lock) syringes.
I always draw BAC with a fresh needle, inject to reconstitute. draw back into syringe after 15 minutes, remove needle, attach syringe filter, reattach needle.
At that point you either inject filtered solution 1) Ideally into a new ultra-spec vial (pyrogen and particle free per FDA standards, Teflon coated stopper prevents industrial rubber chemicals from leaching into peptide solution, which is common with cheap raw butyl rubber), 2) less ideally but still better than not filtering, into original vial or 3) backfill insulin syringe per dose, so...
Ghoul
Member
Surely it needs 2+???
Excuse the dumb questions kinda getting more and more into this genre
No commercial formulation of rHGH has a higher concentration than 15iu/ml without a sophisticated package of excipient ingredients to prevent aggregation. And that 15iu / 1ml is perfectly made pharma.
As a rule of thumb, 12iu/ml is a reasonable limit for UGL. You can fit 3.5ml in one of those little vials.
So 2,5 around thatNo commercial formulation of rHGH has a higher concentration than 15iu/ml without a sophisticated package of excipient ingredients to prevent aggregation. And that 15iu / 1ml is perfectly made pharma.
As a rule of thumb, 12iu/ml is a reasonable limit for UGL. You can fit 3.5ml in one of those little vials.
Ghoul
Member
So 2,5 around that
No, 3ml (I lowered it to 12iu / ml to account for UGL less than pharma standards).
Personally I put 3.5ml in there, because the more, the better in terms of reducing aggregation and keeping more rHGH active.
Keep in mind a lot of 36iu is actually 40-41
Yeah ordered from WWBNo, 3ml (I lowered it to 12iu / ml to account for UGL less than pharma standards).
Personally I put 3.5ml in there, because the more, the better in terms of reducing aggregation and keeping more rHGH active.
Keep in mind a lot of 36iu is actually 40-41
Cheers btw 3 ml it is.
Ghoul
Member
Is there a certain ratio people go for usually? Why not the least amount that you can accurately get in a syringe?
When peptides are too concentrated, they start bumping into each other so often that they stick together forming “aggregates”, and for those like rHGH that aren’t just a string of amino acids but also must be in a specific shape to function, unfold (aka “denature”).
Aggregated or denatured peptides / proteins become inactive, at the least. Worst case scenario they can cause health issues.
In simple terms, too high a concentration makes peptides less stable and more likely to degrade faster.
Here’s an image of aggregates that formed in rHGH in different concentrations*

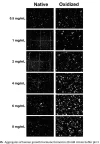
*rHGH that’s damaged (exposed to oxygen in this case), or unfolded (often from heat or shaking/ impact shock / bubbles), starts the aggregation process, and concentration determines how fast and how large (worse) the aggregates become. It’s safe to assume UGL contains damaged rHGH. It’s the root of “cloudy” rHGH issues, but isn’t necessarily visible like it is in that case, since aggregates are usually too small to see. A single visible speck is hundreds of thousands or millions of rHGH. molecules stuck together.
—————
and random excerpts from research papers on the topic because I happen to have them at hand





Last edited:
DamnWhen peptides are too concentrated, they start bumping into each other so often that they stick together forming “aggregates”, and for those like rHGH that aren’t just a string of amino acids but also must be in a specific shape to function, unfold (aka “denature”).
Aggregated or denatured peptides / proteins become inactive, at the least. Worst case scenario they can cause health issues.
In simple terms, too high a concentration makes peptides less stable and more likely to degrade faster.
Here’s an image of aggregates that formed in rHGH in different concentrations*
View attachment 358556View attachment 358553
*rHGH that’s damaged (exposed to oxygen in this case), or unfolded (often from heat or shaking/ impact shock / bubbles), starts the aggregation process, and concentration determines how fast and how large (worse) the aggregates become. It’s safe to assume UGL contains damaged rHGH. It’s the root of “cloudy” rHGH issues, but isn’t necessarily visible like it is in that case, since aggregates are usually too small to see. A single visible speck is hundreds of thousands or millions of rHGH. molecules stuck together.
—————
and random excerpts from research papers on the topic because I happen to have them at hand
View attachment 358549
View attachment 358550
View attachment 358551
View attachment 358554
View attachment 358555
interesting, good to know. Interesting too with the pain being more with more ml, which makes sense, but wonder if that’s even due to the concentration or the literal fact you’re pumping more liquid inWhen peptides are too concentrated, they start bumping into each other so often that they stick together forming “aggregates”, and for those like rHGH that aren’t just a string of amino acids but also must be in a specific shape to function, unfold (aka “denature”).
Aggregated or denatured peptides / proteins become inactive, at the least. Worst case scenario they can cause health issues.
In simple terms, too high a concentration makes peptides less stable and more likely to degrade faster.
Here’s an image of aggregates that formed in rHGH in different concentrations*
View attachment 358556View attachment 358553
*rHGH that’s damaged (exposed to oxygen in this case), or unfolded (often from heat or shaking/ impact shock / bubbles), starts the aggregation process, and concentration determines how fast and how large (worse) the aggregates become. It’s safe to assume UGL contains damaged rHGH. It’s the root of “cloudy” rHGH issues, but isn’t necessarily visible like it is in that case, since aggregates are usually too small to see. A single visible speck is hundreds of thousands or millions of rHGH. molecules stuck together.
—————
and random excerpts from research papers on the topic because I happen to have them at hand
View attachment 358549
View attachment 358550
View attachment 358551
View attachment 358554
View attachment 358555
Ghoul
Member
Damn
interesting, good to know. Interesting too with the pain being more with more ml, which makes sense, but wonder if that’s even due to the concentration or the literal fact you’re pumping more liquid in
It’s both, and excipient ingredients play a role as well.
Just in terms of volume, pharma aims for a max Sub-Q injection of .8ml. Above that it becomes painful for many.
It also changes the speed of absorption.
As injection volume increases (for the same dose), absorption slows down, and blood levels rise more smoothly, up to about 1ml.
This is why high concentrations of GLP drugs, instead of the pharma standard of .5ml, can worsen sides. 1mg in .1ml gets absorbed much more quickly than it does when 1mg is in .5ml. All doses of Tirz (Zepbound) are in .5ml. all doses of Sema (Wegovy) up to 1.4mg are in .5ml, and the two highest, 1.7mg an 2.4mg, are delivered in .75ml to slow down absorption.
Above 1ml SubQ creates a high pressure “bubble”, that stretches tissue walls and high pressure rapidly forces the drug into capillaries.
In the case of rHGH, injections 1ml or larger create a faster, higher GH peak, worsening side effects.
Last edited:
what syringes do you use to inject the bac water and do you need to change the needle every time you draw and extract the water?No, 3ml (I lowered it to 12iu / ml to account for UGL less than pharma standards).
Personally I put 3.5ml in there, because the more, the better in terms of reducing aggregation and keeping more rHGH active.
Keep in mind a lot of 36iu is actually 40-41
Ghoul
Member
what syringes do you use to inject the bac water and do you need to change the needle every time you draw and extract the water?
I use 25g 1” needles and 3ml (luer lock) syringes.
I always draw BAC with a fresh needle, inject to reconstitute. draw back into syringe after 15 minutes, remove needle, attach syringe filter, reattach needle.
At that point you either inject filtered solution 1) Ideally into a new ultra-spec vial (pyrogen and particle free per FDA standards, Teflon coated stopper prevents industrial rubber chemicals from leaching into peptide solution, which is common with cheap raw butyl rubber), 2) less ideally but still better than not filtering, into original vial or 3) backfill insulin syringe per dose, so filtering is done at the last possible moment (controversial I know), since aggregates can “regrow” over time, if, for instance, PH of solution is wrong.
I never reinsert a needle into BAC vial once it’s been in another vial. 25g 1” are around $15 / 100 box.

Ultra Spec Sterile 2ML (13mm neck) Clear Sealed Glass Vials
In order to receive vials in sealed shrink wrap boxes, please order in multiples of 25 Ultra Spec Sterile 2ML-13mm Clear Sealed Glass Vial, Qty 1 ULTRA SPEC sterile depyrogenated vials are assembled with Teflon coated non-latex chlorobutyl rubber stoppers (conforming with USP<381>) and vials...
Last edited:
crispybacon!
Member
I’d recommend not putting the needle back into your bac water vial after you’ve put it in your GH vial to avoid contaminating your bac water.what syringes do you use to inject the bac water and do you need to change the needle every time you draw and extract the water?
I personally use a 3ml syringe with a 1.5” needle and it will hold a little over 3ml (but probably not 3.5). I’d suggest using a 3ml syringe and just accepting that volume, or swap needles, or get a 5ml syringe.
Terminal-Roids
New Member
I’ve been adding 0.9ml to 36iu and injecting .3ml each day (12iu) it’s the highest I’ve gone and it seams to be increasing water retention in hands because I wake up numb, many it would be better to add more water to it but I doubt sides will be any less
Hey sorry to jump in on this!It’s both, and excipient ingredients play a role as well.
Just in terms of volume, pharma aims for a max Sub-Q injection of .8ml. Above that it becomes painful for many.
It also changes the speed of absorption.
As injection volume increases (for the same dose), absorption slows down, and blood levels rise more smoothly, up to about 1ml.
This is why high concentrations of GLP drugs, instead of the pharma standard of .5ml, can worsen sides. 1mg in .1ml gets absorbed much more quickly than it does when 1mg is in .5ml. All doses of Tirz (Zepbound) are in .5ml. all doses of Sema (Wegovy) up to 1.4mg are in .5ml, and the two highest, 1.7mg an 2.4mg, are delivered in .75ml to slow down absorption.
Above 1ml SubQ creates a high pressure “bubble”, that stretches tissue walls and high pressure rapidly forces the drug into capillaries.
In the case of rHGH, injections 1ml or larger create a faster, higher GH peak, worsening side effects.
I was just wondering about volume of a peptide when its lower than .5ml?
I'd stayed on 5mg tirz for a good few months last year and kept the volume at .5ml from your advice and research which was going all well. I had 15mg vials of tirz and it fit well with 1.5ml of bac into the vial. I'm about to get some more i was thinking of getting 30mg vials of tirz because the doses of both 5mg and 7.5mg fit well into that. My only thought though is it would require a 3ml bac to get the volume of .5ml and i'm not sure it would fit in the vial (although maybe it would tbf its probably bigger vials, i think i've just answered my own question haha)
I just wondered though is it a good shout to continue to keep the injection volume at.5ml/would going lower than that make tirz work at a different rate?
Cheers
Most vials will hold 3.5 ml BAC if filled to stopper. 3ml with room to spare.Hey sorry to jump in on this!
I was just wondering about volume of a peptide when its lower than .5ml?
I'd stayed on 5mg tirz for a good few months last year and kept the volume at .5ml from your advice and research which was going all well. I had 15mg vials of tirz and it fit well with 1.5ml of bac into the vial. I'm about to get some more i was thinking of getting 30mg vials of tirz because the doses of both 5mg and 7.5mg fit well into that. My only thought though is it would require a 3ml bac to get the volume of .5ml and i'm not sure it would fit in the vial (although maybe it would tbf its probably bigger vials, i think i've just answered my own question haha)
I just wondered though is it a good shout to continue to keep the injection volume at.5ml/would going lower than that make tirz work at a different rate?
Cheers
wicked thats what i was hoping, cheers for the reply!Most vials will hold 3.5 ml BAC if filled to stopper. 3ml with room to spare.
trynagains
Member
Another thing I do which I find helpful is when adding BAC water to reconstitute I inject with the needle at an angle toward the wall of the vial. This prevents foaming of the solution.I use 25g 1” needles and 3ml (luer lock) syringes.
I always draw BAC with a fresh needle, inject to reconstitute. draw back into syringe after 15 minutes, remove needle, attach syringe filter, reattach needle.
At that point you either inject filtered solution 1) Ideally into a new ultra-spec vial (pyrogen and particle free per FDA standards, Teflon coated stopper prevents industrial rubber chemicals from leaching into peptide solution, which is common with cheap raw butyl rubber), 2) less ideally but still better than not filtering, into original vial or 3) backfill insulin syringe per dose, so filtering is done at the last possible moment (controversial I know), since aggregates can “regrow” over time, if, for instance, PH of solution is wrong.
I never reinsert a needle into BAC vial once it’s been in another vial. 25g 1” are around $15 / 100 box.

Ultra Spec Sterile 2ML (13mm neck) Clear Sealed Glass Vials
In order to receive vials in sealed shrink wrap boxes, please order in multiples of 25 Ultra Spec Sterile 2ML-13mm Clear Sealed Glass Vial, Qty 1 ULTRA SPEC sterile depyrogenated vials are assembled with Teflon coated non-latex chlorobutyl rubber stoppers (conforming with USP<381>) and vials...www.medical-and-lab-supplies.com
Similar threads
- Replies
- 31
- Views
- 328
- Replies
- 4
- Views
- 462

